LipoCube Products Are Now FDA 510k Cleared

We proudly announce that LipoCube medical devices now have the approval of The Food and Drug Administration (FDA) and we are thrilled to inform you that our products are FDA 510k cleared.
BK220767
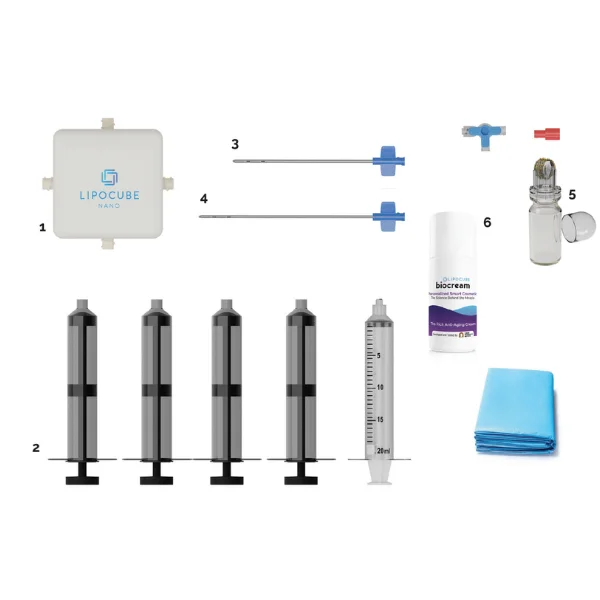
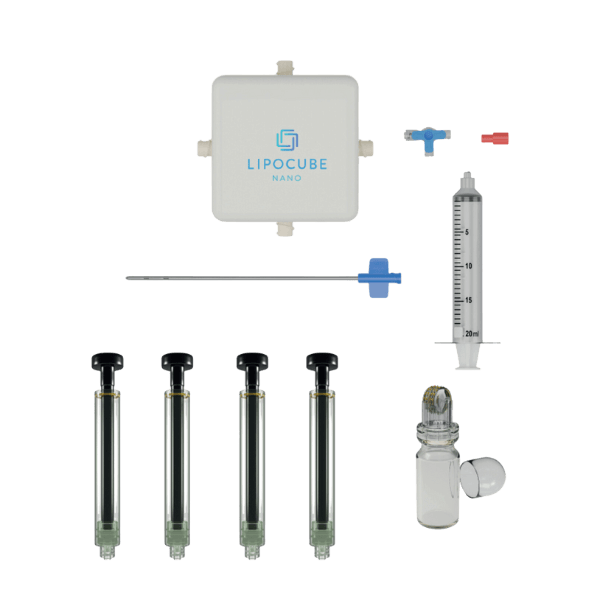
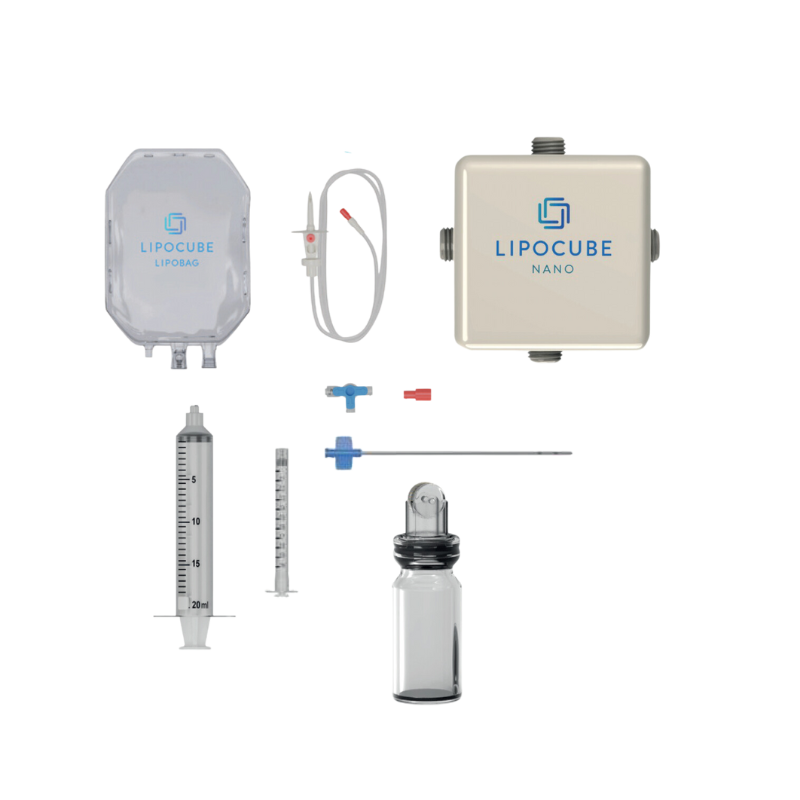
- Trade/Device Name: LipoCube Nano, Liopcube Hybrid I, LipoCube Hybrid II, Centrifuge
Container - Regulation Number: 21 CFR 878.5040
- Regulation Name: Suction lipoplasty system
- Regulatory Class: Class II
- Product Code: QKL
Related Posts
LipoCube products proved by from Brazil’s Health Authority, ANVISA.
December 18, 2023
FUE Europe 10th Meeting Athens Comparison Study
June 15, 2023
LIPOCUBE – 2nd Promising Innovation Award
June 13, 2022
Search
Categories
















Comments are closed.